
 |
||
DMSO Background Literature |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Polar
Agents With Differentiation Inducing Capacity Potentiate
Tumor Necrosis Factor-Mediated Cytotoxicity in Human
Myeloid Cell Lines, Part 3
This article was received February 28, 1994; accepted August 29, 1994.
Besides DMSO, several other highly polar molecules have been described to induce growth inhibition and differentiation in human myeloid cell lines. 25, 26 We tested a group of such agents for potentiation of TNF sensitivity of U937 (Table 4). All compounds were used at concentrations that did not result in any loss of cell viability even upon 96 h of culture (as assessed by trypan blue staining; data not shown). At these doses, all products, except for ethylene glycol and glycerol, induced a growth inhibition with kinetics similar to those observed with 180 mM DMSO (Fig. 1b). The data presented in Table 4 show that most of the agents tested potentiated TNF action, although DMSO seemed to be the most effective in this respect. Table 4 also shows that, in contrast to triethylene glycol which potentiated TNF action, the chemically related ethylene glycol and glycerol were not capable of exerting TNF-sensitizing activities on U937 cells. Of the agents capable of potentiating TNF actions, there were large differences in the molar concentrations necessary for antiproliferative and/or TNF-potentiating effect. In this respect, the presence of methyl groups seems to be very important: the more methyl groups are present, the Iower are the concentrations needed to induce growth inhibition and potentiation of TNF action. Similar results were obtained for the myelomonocytic THP1 cell line (data not shown). The K562 cell line, which could not be rcndered TNF-sensitive by DMSO (Table 1), also could not be sensitized by the drugs listed in Table 4.
Effect of Pretreatment with Different Polar Agents on TNF Sensitivity of U937 Cells
To investigate whether an augmented TNF response in DMSO-pretreated myelold cells was related to an upregulation of TNF receptor expression, we compared the effect of a 24-h treatment with 180 mM DMSO with respect to the binding of iodinated TNF on U937, U937r, or THP1 cells. Surprisingly, all cell lines were found to have lost part of their TNF binding capacity upon DMSO incubation (Fig. 4; data for U937r are not shown). Scatchard analysis revealed that this effect was due to a decrease in receptor number and not to a change in ligand affinity.
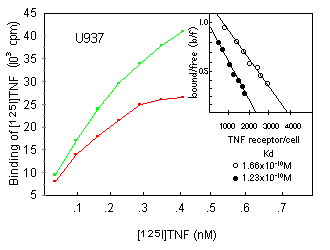 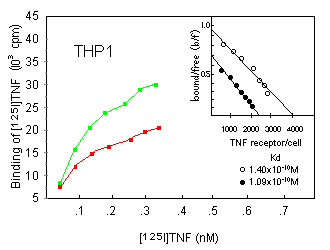
We further investigated whether DMSO similarly affected p55 and p75 TNF receptors, both of which are expressed on U937 cells (as assessed by flow cytometric analysis; data not shown). This was done by the use of anti-TNF receptor mAbs of the htr and utr series, respectively; htr mAbs recognize the p55 TNF receptor, whereas utr mAbs specifically interact with the p75 TNF receptor. 10 htr9 and utrl mAbs, separately or in combination, were used to block either one or the other of both TNF receptor types before addition of iodinated TNF (Fig. 5). The relative amount of TNF still bound was found to be unaltered after DMSO treatment, suggesting that DMSO treatment reduced p55 and p75 receptor expression similarly. Similar results were obtained receptor expression with U937r cells (data not shown).
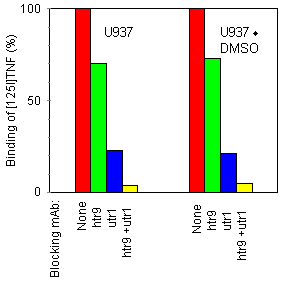 Figure 5. Effect of anti-TNF receptor mAb on [125I]TNF binding. U937 and U937·DMSO cells were incubated at 37°C with the indicated mAb at 20 Because some investigators have attributed enhanced TNF responsiveness of cells to enhanced internalization or degradation of TNF, 46, 47 we investigated these processes in U937 and U937·DMSO cells (Fig. 6). No significant difference was observed concerning kinetics of internalization: receptor-bound radioiodinated TNF disappeared from the cell surface of U937 and U937·DMSO at a similar rate, correlating with a concomitant intracellular uptake. TNF was extensively degraded in both cell types. The amount of dissociated, undegraded TNF in the culture medium was identical for U937 and U937·DMSO cells, indicating that DMSO did not influence the strength of the interaction between TNF and its receptor (data not shown).
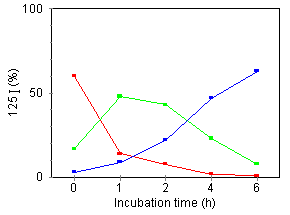
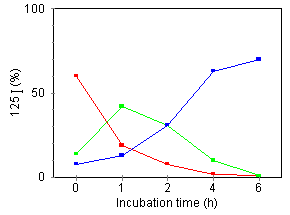
Figure 6. Effect of DMSO on internalization and degradation of cell-bound [125I]TNF. [125I]TNF was incubated for 2 h at 4°C with U937 (left) or U937·DMSO (right) cells. Subsequently, cells were incubated at 37°C and, at the indicated times, the radioactivity of surface-bound (red), internalized (green), and degraded TNF released into the medium (blue), were measured as described in Materials and Methods. The percentage of [125I]TNF recovered from each fraction analyzed was plotted. Data represents the average of three determinations (coefficient of varience < 10 %).
A recent report proposes that a functional form of TNF is a membrane-embedded trimer, the effects of which are mediated through a putative ion channel-forming activity. 31 As DMSO is known to be able to exert changes in cell membrane characteristics,32 we investigated whether there could be a correlation between enhancement of TNF effects by DMSO pretreatment and TNF insertion in cellular membranes. htr9 mAb has been shown to have agonistic activities through binding to the p55 TNF receptor. 48 We observed that this mAb also behaved as an agonist with respect to the induction of cytostasis/cytotoxicity in our model system (Figure 7). Non-DMSO-treated U937 cells were not affected by this mAb (data not shown). Cross-linking the receptor-bound antibody with antimouse IgG neither enhanced the cytotoxicity in U937·DMSO cells, nor induced any cell death in the parental U937 cells (data not shown). The htr9 mAb is structurally unrelated to TNF and thus cannot form transmembrane channels as described for TNF. 31 These results provided a first indication for receptor-mediated and not insertion-mediated TNF signalling. In contrast with htr9, the p75-specific utrl mAb was not able to transduce an antiproliferative signal, even after DMSO pretreatment (Figure 7) or after cross-linking of the p75-bound mAb (data not shown). Analgous results have been obtained by others. 10, 48 These data show that p55 TNF receptor is responsible for mediating the TNF-induced cytotoxicity in U937·DMSO cells.
In a second approach, we tested the effect of TNF mutants that have decreased p55 TNF receptor binding capacity, but have retained the physico-chemical and conformational charachteristics of wild type TNF. 35 It would be expected, therefore, that these TNF mutants could insert into cell membranes in a similar way as wild type TNF. This would result in an identical cytotoxicity as of wild type TNF, if membrane insertion is involved in the cell killing of U937 after DMSO pretreatment. The TNF mutants, arranged in order of decreasing p55 TNF receptor binding capacity, showed a concomitantly decreasing cytotoxic activity on the human HEp2 adenocarcinoma cell line (Table 5), on which the effect of TNF has been demonstrated to be p55 TNF receptor-mediated. 10 When tested on U937 cells pretreated for 24 h with DMSO, a decrease in TNF bioactivity was observed similar to that on HEp2 cells (Table 5). These data indicate that TNF receptor interaction and not membrane insertion is responsible for TNF action on DMSO-treated U937 cells. Bioactivity
of TNF Mutants on HEp2 and U937·DMSO Cells
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
© 2001-2022
All rights reserved |