
 |
||
DMSO Background Literature |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Polar
Agents With Differentiation Inducing Capacity Potentiate
Tumor Necrosis Factor-Mediated Cytotoxicity in Human
Myeloid Cell Lines, Part 2
This article was received February 28, 1994; accepted August 29, 1994.
Characterization of growth arrest and cell death of U937 cells following combined DMSO/TNF treatment Several investigators have demonstrated that treatment of human leukemic cells with DMSO is associated with growth inhibition and induction of differentiation. 24, 27 Incubation of our U937 cell line in the presence of DMSO induced a time- and concentration-dependent reduction of the cells' proliferative capacity: over an assay period of 72 h and at 210 mM DMSO, cells were fully growth-inhibited (Fig. 1A). As inhibition of thymidine incorporation can reflect both cytostatic as well as cytotoxic effects, we also investigated cell viability by means of trypan blue exclusion and the colorimetric MTT assay. Fig. 1B shows that treatment with 210 mM DMSO, a dose inducing nearly complete growth inihibition, did not induce any cytotoxicity: total cell viability as measured by trypan blue, remained constant over an assay period of 72 h. Furthermore, at this time point, over 85% of the cells in culture could be stained with MTT (data not shown). Higher DMSO doses (280 mM) became cytotoxic. In further experiments, concentrations of DMSO used were maximally 180 mM. 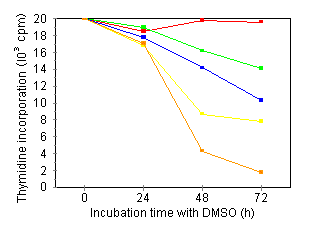 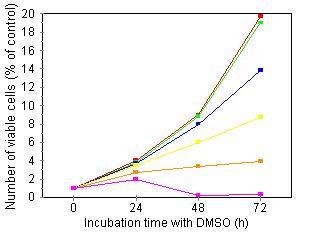 Treatment of U937 with 50 pg/ml TNF for 24 h did not induce growth inhibition or cell death (Fig. 2A). However, upon 24-h pretreatment with increasing doses of DMSO, TNF-induced growth inhibition and cell death became apparent within a 24 h period (Fig. 2A). The observed TNF activity could be blocked completely by neutralizing antiserum to human TNF (data not shown).
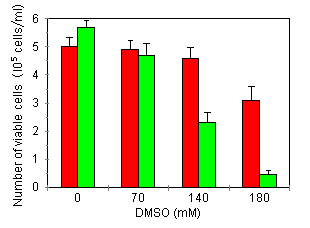
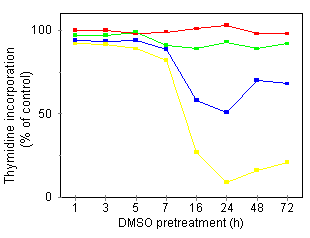
Besides a DMSO concentration dependency, there was also a need for a prolonged preincubation period: restricted DMSO pretreatment times (less than 7 h) were not sufficient to render the cells susceptible to TNF (Fig. 2B). At 180 mM DMSO, priming of the U937 cells was optimal after a 24-h continuous incubation with DMSO, a time point at which antiproliferative effects of DMSO alone were not yet apparent (Fig. 1A). In further experiments, U937 cells, pretreated for 24 h with 180 mM DMSO, will be designated as U937·DMSO cells. Potentiation of TNF cytotoxicity by DMSO in myeloid cell lines with differing degrees of maturation We investigated whether DMSO also altered the TNF sensitivity of established human myeloid cell lines other than U937. These included (classified by increasing degree of maturity): erythroleukemic K562, myeloblastic KG1, promyelocytic HL60, monoblastic (myelomonocytic) THP1, and monocytic MonoMac6 cell lines. As for U937 cells, treatment with 180 mM DMSO for 72 h induced inhibition of cell growth, as measured by the MTT colorimetric assay, in KG1, HL60, and THP1 without loss of cell viability (Table 1, data for KG1 not shown). The MonoMac6 cell line, 180 mM, but not 90 mM, was cytotoxic. In K562 cells, only modest antiproliferative effects were observed, even following 72 h of culture in the presence of 180 mM DMSO. Sensitivity to TNF alone of the indicated cell lines largely differed, at least when assayed over long periods. After 72 h of incubation in the presence of 10 ng/ml TNF, all three monoblastic/monocytic cell lines (U937, THP1, and MonoMac6; Table 1) as well as the KG1 cell line (data not shown) showed diminished cell growth, in contrast to K562 and HL60 cell lines. The absence of a TNF effect on both K562 and HL60 cells was not due to the absence of p55 or p75 TNF receptors, as both receptor types were detected on these cell lines by flow cytometry analysis (data not shown). Finally, we tested whether DMSO pretreatment and/or cotreatment influenced the TNF sensitivity of these cell lines. In pretreatment experiments, cells were incubated for 24 h with 180 mM DMSO, followed by 24 h incubation with TNF (Table 2). DMSO only up-regulated the responsiveness of cell lines which already displayed TNF susceptibility as such in long term assays (KG1, U937, THP1, and MonoMac6). The potentiating effect was most obvious in the case of U937 and THP1 cells. Similar observations were made when cell viability was measured by trypan blue exclusion (data not shown). We also tested U937-derived cell lines which were resistant to TNF cytotoxicity. This resistance was not due to the absence of p55 or p75 receptors (whose expression was not significantly different from parental U937 cells as detected by flow cytometric analysis; data not shown), nor to the absence of TNF internalization and degradation (data not shown). As expected, U937r cells did not show any growth inhibition when cultured in the presence of 10 ng/mi TNF for 72 h (data not shown). However, these U937r cells could be sensitized to TNF cytotoxicity by DMSO pretreatment (Table 2). In contrast to U937 cells, however, about 100 times higher TNF doses were needed to obtain this effect.
Effect of DMSO Cotreatment on TNF Sensitivity of Various Human Myloid Cell Linesa
Effect of DMSO Pretreatment on TNF Sensitivity of Various Human Myelold Cell Linesa
With cotreatment with DMSO and TNF, we obtained potentiating effects of DMSO on TNF-induced cytostasis/cytotoxicity in U937, THP1, and MonoMac6 cells, whereas the K562 cell line remained unsusceptible to TNF, even after 72 h (Table 1). Furthermore, in contrast with pretreatment results, cotreatment of the HL60 cell line with DMSO and high doses of TNF (10 ng/ml) for 72 h resulted in the induction of TNF sensitivity. Finally, it is important to note that in all cell lines tested, except for K562, DMSO could also induce or potentiate TNF-induced cytostasis/cytotoxicity at a concentration which, on its own, had no antiproliferative effect, even after 72 h (120 mM, Table 1).
Table 3 summarizes the results of a representative experiment in which the TNF sensitivity of several non-myeloid cell lines was tested, either in the absence or presence of DMSO, in a 72-h MTT viability assay. First, DMSO alone reduced the amount of cells in all cell lines in comparison with control cult9A6, MCF-7, PC60 TR55/75) TNF sensitivity. Potentiation of TNF cytostasis/cytotoxicity by DMSO was not found in any of the non-myeloid cell types tested and thus, DMSO-induced potentiation was restricted to myelold cell lines. Effect of DMSO Cotreatment on TNF Sensitivity of Non-Myelold Cells
It has been reported that TNF can cause DNA degradation before cell lysis. 45 Agarose gel electrophoresis of total cellular DNA revealed that 24-h treatment of U937 cells with 180 mM DMSO did not induce DNA degradation, in contrast to a 4-h treatment with 10,000 pg/ml TNF which induced some apoptotic DNA degradation (Fig. 3). Apoptosis or programmed cell death is characterized by internucleosomal cleavage of the DNA resulting in the appearance of oligonucleosome fragments consisting of 200 basepairs or multimers of it. It is remarkable that 10,000 pg/ml TNF induced some apoptotic DNA degradation in U937 cells within 4 h while this TNF dose only induced small cytostatic/cytotoxic effects within 24 h (Table 1). DMSO pretreatment was found to potentiate TNF-induced DNA fragmentation in U937, resulting in almost complete DNA degradation at 10,000 pg/ml TNF. Enhancement of TNF-induced DNA fragmentation by DMSO was also observed in THP1 and HL60 cell lines (data not shown). In the TNF-resistant U937r cell line, TNF induced DNA fragmentation only upon pretreatment with DMSO (Fig. 3). Thus, in myeloid cells there was a good correlation between the potentiation of TNF action by DMSO and the occurrence of TNF-induced fragmentation. This observation probably indicates that TNF potentiation by DMSO was restricted to cells with a propensity for apoptotic cell death. However, a TNF-induced ladder pattern of DNA digestion was also observed in some non-myelold cells (24T2·5, WEHI 164 cl13, KYM39A6, PC60 TR55/75), whose TNF sensitivity was either inhibited or remained unaltered upon DMSO cotreatment (see summary in Table 2). Thus, apoptotic DNA digestion does not predict a propensity for DMSO to potentiate TNF cytotoxicity.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
© 2001-2022
All rights reserved |