
 |
||
DMSO Background Literature |
|||||||||||||||
|
M. Steven Evans,a, Kenneth H. Reid,b, and James B. Sharp Jr.c Received June 29, 1992; Revised version received November 3, 1992; Accepted November 5, 1992.
Dimethyl sulfoxide (DMSO) is readily absorbed through skin, and relieves musculoskeletal pain when applied topically to painful areas. We studied the effects of DMSO on C-type nerve fibers, which mediate pain sensation. DMSO was applied directly to exposed cat sural nerves. C fiber conduction velocity was slowed by DMSO, even in low concentrations (5-7% v/v). Higher concentrations completely blocked C fiber conduction, with a minimum blocking concentration of 9%. Onset of nerve block was almost immediate with 15% DMSO or higher concentrations. C fiber blockade may account for analgesia with DMSO.
Dimethyl sulfoxide (DMSO) is a solvent with local analgesic properties. It penetrates skin quickly,11 so for analgesia it is applied directly to skin over the painful area. Initial reports on DMSO emphasized its usefulness as a local analgesic,10 and it became a popular remedy for relief from the musculoskeletal pain of arthritis, sprains and strains,13 even though never approved by the Food and Drug Administration for this use. 7 Clinical studies of DMSO found it to be analgesic,2, 4, 12, 14 although some studies found no benefit. 16 Somatic pain is mediated by unmyelinated C type nerve fibers terminating in the skin, muscle and joint capsules. 5 We have studied a possible mechanism of DMSO-induced analgesia by observing the effects of DMSO on conduction in C fibers of cat cutaneous nerves. (Some of these results were presented previously in abstract form.) Ninety percent DMSO in water (Eqigisic, Burlington Bio-Medical Co.) was diluted to the desired concentration (expressed as v/v) with deionized water and Hank's Balanced Salt Solution concentrate (Grand Island Biological Company). The final concentrations were (in mM):
The solute concentration was 280 mM, but total osmolarity was greater due to the DMSO (1588 mosmol for a 10% DMSO solution). If necessary, the solution was titrated to pH 7.4 with small amounts of NaH2CO3. Sural nerves of twenty-five random source 2-4 kg cats were studied. Cats were anesthetized with 40 mg/kg sodium pentobarbital intravenously, supplemented as needed to maintain a level of anesthesia sufficient to eliminate withdrawal reflexes. The left sural nerve and its epineurial sheath were carefully microdissected from the underlying muscle and fascia, but left in situ. Proximally, the sural nerve remained connected to the sciatic nerve, and distally remained covered by fascia overlying the gastrocnemius muscle. The nerve's blood supply was left intact. The nerve was placed in a small bath, a slotted glass cylinder with an internal length of 2.2 cm. Bathing medium was injected into the nerve bath manually with a syringe, and removed at a rate of 0.08 ml/min with an infusion pump. The volume of the nerve bath was 0.4 ml, so that a complete change of solution took 5 minutes. A bipolar stimulating electrode was placed on the nerve proximal to the bath, and a bipolar recording electrode distal to it (antidromic stimulation). The cat limb, electrodes and bath were then enclosed in a plastid tent kept at 100% humidity, 36°C. Stimulating and recording electrodes were fabricated from chlorided silver wires. Constant-voltage capacitance-isolated square wave stimuli, 0.5 ms duration, were given at 0.3-3 Hz. Stimulus intensity was set to twice the voltage at C fiber threshold, which ranged from 5 to 10 V (mean of 7.5 V ± 0.37 S.E.M.). C fiber responses were identified by their slower conduction velocity, smaller amplitude and higher stimulus threshold compared to A fibers. Conventional electronic devices were used for recording and displaying responses. A general-purpose microcomputer averaged 16 responses, which were then plotted with a chart recorder. The gain and timebase needed to display C responses were incompatible with simultaneously recording A fiber responses.
Sural Nerve Responses 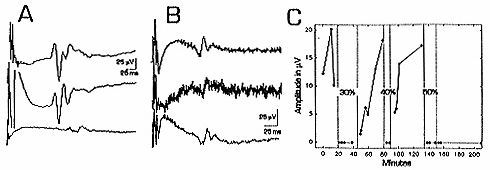
A: sural nerve responses are stable. Top: C fiber waveform at beginning of experiment. A fiber responses at the beginning of the record are distorted because of the high gain (which saturates the signal averager) and slow timebase needed to demonstrate C responses. Positive is upward in all traces. Middle: response 9 h later, with no DMSO treatment. Bottom: same nerve, C fiber response blocked 10 min after application of 10% DMSO. B: DMSO transiently increases spontaneous firing. Top: control sural nerve C fiber response. Middle: 1 min after 5% DMSO, background noise is dramatically increased by spontaneous action potential firing. Bottom: after 20 min in 5% DMSO, spontaneous firing is not apparent. C: DMSO blockade is reversible.
A nerve was exposed to DMSO during the times indicated
by dotted lines. Control responses (0-20 min) varied
in amplitude. 30% and 40% DMSO quickly abolished C fiber
responses, with the blockades reversed by washing. In
this example, 50% DMSO caused irreversible blockade.
C fiber compound action potential amplitude (most positive
point minus most negative point) and latency (to most
positive point) were measured. In agreement with previous
investigators,5, 6
we found that conduction velocity was stable, but C
fiber response amplitudes and shapes varied from minute
to minute. Because of this, we used complete block of
C fiber responses as an endpoint (Fig.
1A), since this never occurred normally. Complete
block was defined as loss of any organized trace of
the original waveform. The sural nerve preparation was
stable in long-term experiments. Two nerves were followed
without drug treatment for nine hours, with no systematic
changes in response amplitude or latency (Fig.
1A). During experiments, the health of the nerve
was assessed by stroking hairs on the foot, causing
action potentials to fire. In no case was responsiveness
to this natural stimulus diminished, even after complete
blockade of nerve conduction through the bath. Experiments were performed by increasing the DMSO concentration
until C fibers were blocked, then lowering the concentration
until the block reversed. Immediately after DMSO application,
a 1-5 min transient period of increased noise was always
seen with concentrations of 5% or more (Fig.
1B). This was due to asynchronous spontaneous firing
of axons, transiently reducing the amplitude of evoked
C fiber responses. DMSO effects on C fiber response amplitudes were concentration-dependent.
Five percent and 7% DMSO tended to reduce C fiber response
amplitudes, but the minimum blocking concentration was
9% (Figs. 1A, 2B,
and C). In 14 experiments using
9% or 10% DMSO, C fiber responses were invariably reduced,
and were completely blocked in 9 experiments (Figs.
1A and 2A). In these 9 experiments,
blockade occurred 22.0 min ± 2.0 after DMSO application.
DMSO concentrations of 15% or more reliably blocked
C fibers. All nerves exposed to 15% or more DMSO were
quickly blocked (mean 6.3 minutes ± 0.3, n
= 15 experiments) (Fig. 2B). Five percent DMSO relieved block caused by higher concentrations
(Fig. 2A), but reversal of established
blockade was never seen with 9% DMSO or higher concentrations.
In 11 experiments (8 nerves), C fiber blockade was produced
by 9% to 26% DMSO, followed by application of 5%; recovery
was good in 10 of the 11 experiments. Because blockade
was usually reversible, two or three experiments were
performed on some nerves that recovered to 75% of their
maximal pre-DMSO response amplitude. Six nerves were exposed to low concentrations of DMSO
for several hours (not illustrated). With such prolonged
exposure, 1% to 7% DMSO blocked four nerves 6.25-8 h
after application of drug, suggesting that long exposure
to low concentrations of DMSO can reduce C fiber transmission.
In three of the four nerves, blockade reversed after
extended washing.
DMSO reduces C fiber response amplitude and conduction velocity. DMSO reduced C fiber conduction velocity (Fig. 2D- F). Concentrations as low as 2% DMSO produced definite increases in response lattency. Unlike the effect on response amplitude, which took tens of minutes to develop with 9% DMSO, the effect on response latency was almost instantaneous. This effect was stable during the time of drug exposure, and reversed after drug removal. Response latency increased by almost 200% with 10% DMSO, implying that overall conduction velocity in the nerve segment between stimulating and recording electrodes was reduced by 50%. However, this represents a conservative estimate of the effects of DMSO, since the nerve segment studied, usually 5 cm long, was exposed to DMSO only within the 2.2 cm long nerve bath. The mechanism of the conduction block induced by DMSO may involve potassium channel blockade. Sawada and Sato18 found that DMSO caused a rapid membrane depolarization and decrease in membrane conductance of Aplysia neurons, consistent with blockade of leak potassium channels. DMSO slowed action potential repolarization, and voltage clamp study showed that DMSO inhibited the delayed rectifier potassium channel. Their study implies that DMSO can produce a depolarizing nerve block through inhibition of both voltage-sensitive and voltage-insensitive potassium channels. Depolarizing block is consistent with results of the present experiments, in which the transient increase in electrical noise seen immediately after application of DMSO suggests that fibers have depolarized sufficiently to allow spontaneous action potential firing. How DMSO might specifically affect potassium channels is not clear, but Mayer and Avi-Dor15 have suggested that DMSO can change the state of hydration of the potassium ion, which might alter its ability to penetrate ion channels. The mechanism of DMSO's ability to profoundly reduce C fiber conduction velocity is unclear. This effect is fast, stable, and reversible. It is detectable with low concentrations of the drug. Conduction velocity reduction may also be caused by effects on potassium channels. One possibility is that persistent membrane depolarization could facilitate sodium channel inactivation, with consequent slowing of action potential generation. A second possibility is that an increase in membrane resistance due to blockade of potassium channels might affect conduction velocity, but in this case the magnitude and direction of the effect are not easily predicted. 9 Conduction velocity could also be reduced by increasing extracellular electrical resistance,8 but under normal conditions, conduction velocity changes appreciably only with manyfold increases in extracellular resistance. The ability of 9% DMSO to block C fibers contrasts with previous studies on myelinated A type nerve fibers, in which much larger concentrations were needed. Davis et al. 3 found that 50% DMSO was necessary to block muscle twitch in a frog sciatic nerve-gastrocnemius muscle preparation. Becker1 found that 75% DMSO blocked both A and C fibers. Shealey19 studied a prolonged small fiber afterdischarge in spinal cord, medulla and tegmentum following stimulation of the superficial radial and sural nerves, and found it to be blocked by exposing the peripheral nerve to 5-10% DMSO, a result that could be due to blockade of C fibers. The present experiments suggest that DMSO can be an effective topical analgesic. If analgesia with DMSO depends on blockade of C fibers, one requirement is that the drug concentration around C fibers leading from the painful area be brought to 10% or more for several minutes. It is likely that this situation occurs clinically, since DMSO penetrates skin easily, and topical application of 70-90% is commonly used. Insofar as DMSO selectively affects somatic C fibers, a selective analgesia and loss of temperature sensation, rather than complete anesthesia, may be expected. Lightly-myelinated A-delta fibers that mediate sharp (first) pain were not investigated in this study, but if unaffected by DMSO, would allow sharp pain to persist, and deep, aching pain to be abolished. Deep, aching pain is present in arthritis and musculoskeletal injuries, the conditions for which DMSO has often been used. This work is part of a thesis submitted by M.S.E. to the Graduate School of the University of Louisville, August, 1984. The authors thank Jennifer Evans and Dean Naritoku for critical reading of the manuscript. References
aM. Steven Evans, Division of Neurology, Department of Internal Medicine, Southern Illinois University School of Medicine, Springfield. IL (USA). bKenneth H. Reid, Department of Anatomy and Neurobiology, University of Louisville School of Medicine, Louisville, KY (USA). cJames B. Sharp Jr., Department of Veterinary Medicine, University of Louisville School of Medicine, Louisville, KY (USA).
Neuroscience Letters, Volume 150, 1993, pp. 145-148., Elsevier Scientific Publishers, Ireland Ltd. Direct all correspondance to: M.S. Evans, Box 19230, Division of Neurology, Southern Illinois University School of Medicine, Springfield, IL 62794, USA. Fax: (1) (217) 788-5567. DMSO Organization wishes to thank Elsevier Scientific Publishers for allowing this article to be placed on our World Wide Web site. The publisher retains all copyright. To copy any portion of this article, please obtain permission from the publisher. |
|||||||||||||||
© 2001-2022
All rights reserved |