J. Shupack, M. Stiller, L Davis, C Kenny, L. Jondreau
Section of Dermatopharmacology • Department of Dermatology • New York University Medical Center
New York, N.Y.
Received: February 21, 1991
The recent in vivo appearance of acyclovir-resistant strains of
herpes simplex virus stresses the need for new therapeutic agents to combat
this common virus. Topical interferon preparations may help fill this
void. In the present study dimethyl sulfoxide was combined with ![]() -interferon
in an ointment base to increase percutaneous penetration. In this double-blind,
placebo-controlled trial, patients with recurrent genital herpes simplex
using the topical
-interferon
in an ointment base to increase percutaneous penetration. In this double-blind,
placebo-controlled trial, patients with recurrent genital herpes simplex
using the topical ![]() -interferon
preparation had a more rapid cessation of viral shedding when compared
to the placebo group (66% culture negative on day 1 vs. 25% of placebo
patients; p<0.02). In the 90-day post-treatment period, interferon-treated
patients had fewer recurrences than their placebo-treated counterparts
(1.18 vs. 2.25). This reduction while not statistically significant was
encouraging, (P<0.10).
-interferon
preparation had a more rapid cessation of viral shedding when compared
to the placebo group (66% culture negative on day 1 vs. 25% of placebo
patients; p<0.02). In the 90-day post-treatment period, interferon-treated
patients had fewer recurrences than their placebo-treated counterparts
(1.18 vs. 2.25). This reduction while not statistically significant was
encouraging, (P<0.10).
Recently, the incidence of genital herpes simplex infections in the United States has risen dramatically.1 There are an estimated 260,000-500,000 cases per year, and genital herpes simplex virus (HSV) infections are a source of significant physical and psychological morbidity.2
Clinical studies in patients with recurrent genital herpes simplex have
demonstrated a decrease in frequency and shortened duration of episodes,
as well as a more rapid healing of lesions in those receiving oral acyclovir
versus placebo. Oral acyclovir has been shown to safely
decrease the frequency of episodes when taken daily for periods up to
3 years.3-10 Straus et al.11
recently reported that chronic suppressive oral acyclovir failed to reduce
the rate of asymptomatic viral shedding. It is potentially significant
that these investigators cultured acyclovir-resistant strains of HSV from
asymptomatic, nonimmunocompromised hosts with increased frequency.Interferon,
a glycoprotein with antiviral and antineoplastic activity, was first shown
to be beneficial in preventing reactivation of herpes labialis in patients
undergoing microneurological decompression of the trigeminal nerve. ![]() -Interferon
administered intramuscularly prior to operation significantly reduced
viral shedding and new lesion formation in these patients.12We
recently reported that topical
-Interferon
administered intramuscularly prior to operation significantly reduced
viral shedding and new lesion formation in these patients.12We
recently reported that topical ![]() -interferon
(at a concentration of 106 IU/g in a nonoxynol-9 gel base) was more
effective than placebo in a double-blind, placebo-controlled clinical
trial in patients with recurrent genital HSV. (Nonoxynol-9 is a widely
used, safe surfactant which aids in the uniform dispersion of interferon
in the vehicle.13 The duration of viral shedding and
the time until the end of all subjective symptoms including pain, burning
and itching were significantly reduced in patients receiving the interferon
preparation.14 Friedman-Kein et al.15
using a similar topical
-interferon
(at a concentration of 106 IU/g in a nonoxynol-9 gel base) was more
effective than placebo in a double-blind, placebo-controlled clinical
trial in patients with recurrent genital HSV. (Nonoxynol-9 is a widely
used, safe surfactant which aids in the uniform dispersion of interferon
in the vehicle.13 The duration of viral shedding and
the time until the end of all subjective symptoms including pain, burning
and itching were significantly reduced in patients receiving the interferon
preparation.14 Friedman-Kein et al.15
using a similar topical ![]() -interferon
preparation reported favorable results. In their placebo-controlled study
there was a decrease in viral shedding, time to scabbing and formation
of new lesions in the group of patients who received the
-interferon
preparation reported favorable results. In their placebo-controlled study
there was a decrease in viral shedding, time to scabbing and formation
of new lesions in the group of patients who received the ![]() -interferon.
Several clinical trials by Israeli investigators have evaluated topical
human fibroblast (
-interferon.
Several clinical trials by Israeli investigators have evaluated topical
human fibroblast (![]() )-interferon
in the treatment of recurrent genital and facial HSV infections. These
investigators reported not only a decrease in the duration of episodes
and severity of symptoms but also a decrease in the subsequent recurrence
rate.16-18 Topical acyclovir in an aquaeous propylene
glycol cream base has shown significant efficacy in the treatment of recurrent
genital HSV infections in controlled clinical trials.19-22
On the other hand, 5% acyclovir in a polyethylene glycol ointment base,
the topical preparation which is commercially available in the United
States, has failed to show significant clinical efficacy in double-blind
studies.23-26 Furthermore, topical acyclovir use has
failed to alter the frequency of recurrences or the amount of time between
recurrences.27-28In the present study
)-interferon
in the treatment of recurrent genital and facial HSV infections. These
investigators reported not only a decrease in the duration of episodes
and severity of symptoms but also a decrease in the subsequent recurrence
rate.16-18 Topical acyclovir in an aquaeous propylene
glycol cream base has shown significant efficacy in the treatment of recurrent
genital HSV infections in controlled clinical trials.19-22
On the other hand, 5% acyclovir in a polyethylene glycol ointment base,
the topical preparation which is commercially available in the United
States, has failed to show significant clinical efficacy in double-blind
studies.23-26 Furthermore, topical acyclovir use has
failed to alter the frequency of recurrences or the amount of time between
recurrences.27-28In the present study ![]() -interferon
was combined with dimethyl sulfoxide (DMSO). DMSO, a dipolar
aprotic solvent, has been shown in clinical studies to be effective
in enhancing the penetration of topical medications.29-31We
report the results of a study comparing the efficacy and safety of a topical
-interferon
was combined with dimethyl sulfoxide (DMSO). DMSO, a dipolar
aprotic solvent, has been shown in clinical studies to be effective
in enhancing the penetration of topical medications.29-31We
report the results of a study comparing the efficacy and safety of a topical
![]() -interferon
ointment which also contained DMSO in the treatment of recurrent genital
herpes.
-interferon
ointment which also contained DMSO in the treatment of recurrent genital
herpes.
The study population consisted of 40 immunocompetent men and women, aged
18 years or older, who had had at least two episodes of recurrent genital
herpes simplex within the past 6 months. Diagnosis was confirmed by viral
culture or by a dermatologist's clinical evaluation. All patients agreed
to discontinue the use of topical and systemic medications intended for
treatment of herpes simplex or which might alter symptoms such as pruritus
or pain, e.g. antihistamines and analgesics, 30 days prior to enrollment
and for the duration of the study. A phisical examination and medical
history were obtained on all subjects prior to enrollment. This clinical
trial was approved by the Institutional Board of Research Associates of
New York University Medical Center prior to its initiation, and an informed
consent was obtained from all participants in accordance with Food and
Drug Administration guidelines. This clinical trial was double-blinded
with a placebo control. The patients were given identically labeled tubes
of either 105 IU/g ![]() -interferon
in hydrophilic ointment with 5% DMSO or hydrophilic ointment with 5% DMSO,
which served as a placebo control. The decision to use a lower concentration
of interferon than was used in our earlier clinical trial 14
was based on consideration of the more penetrating DMSO-containing vehicle
in the present study. Human leukocyte interferon was prepared by Vira-Tech
Inc. The ointment base contained water, petrolatum, mineral oil, mineral
wax, wool alcohol, 2-bromo-2-nitropropane-1,3-diol, benzyl alcohol, carboxymethyl
cellulose and 5% DMSO.The patients were interviewed at a time when they
were not having a herpetic outbreak and instructed to begin applying the
ointment four times a day at the onset of the first prodromal symptoms
of their next attack. All patients were then examined at our institution
within 24 h of the onset of that episode of herpes simplex (day 1)
and again on days 3, 5, and 7. Outbreaks were treated until resolution
or up to a maximum of 7 days. Subsequent recurrences were not treated.
Additionally, all participants were required to make a follow-up visit
to NYU Medical Center 60-90 days after the day 5 visit. Additionally,
during the 6-month period after cessation of therapy, the study subjects
were expected to keep a diary recording the number of herpetic episodes
they had and the duration of those recurrences. This information was obtained
from the study subjects in a 6-month post-treatment phone interview conducted
by one of the study coordinators. During all visits herpetic lesions were
morphologically staged (as papules, vesicles, crusts, or healed), lesion
counts and measurements taken, and patients were asked to rate their subjective
symptoms (which included pain, pruritus, burning, and stinging). Patient
serum was collected on day 3 to determine serum electrolytes, liver function
tests, hematologic profiles as well as DMSO levels. DMSO serum levels
were measured by gas chromatography at the National Medical Services Laboratory,
Willow Grove, Pa., USA. Specimens for virus isolation were taken on days
1, 3, and 5 by the methods used by Friedman-Klein et al.15Statistics
were prepared by
-interferon
in hydrophilic ointment with 5% DMSO or hydrophilic ointment with 5% DMSO,
which served as a placebo control. The decision to use a lower concentration
of interferon than was used in our earlier clinical trial 14
was based on consideration of the more penetrating DMSO-containing vehicle
in the present study. Human leukocyte interferon was prepared by Vira-Tech
Inc. The ointment base contained water, petrolatum, mineral oil, mineral
wax, wool alcohol, 2-bromo-2-nitropropane-1,3-diol, benzyl alcohol, carboxymethyl
cellulose and 5% DMSO.The patients were interviewed at a time when they
were not having a herpetic outbreak and instructed to begin applying the
ointment four times a day at the onset of the first prodromal symptoms
of their next attack. All patients were then examined at our institution
within 24 h of the onset of that episode of herpes simplex (day 1)
and again on days 3, 5, and 7. Outbreaks were treated until resolution
or up to a maximum of 7 days. Subsequent recurrences were not treated.
Additionally, all participants were required to make a follow-up visit
to NYU Medical Center 60-90 days after the day 5 visit. Additionally,
during the 6-month period after cessation of therapy, the study subjects
were expected to keep a diary recording the number of herpetic episodes
they had and the duration of those recurrences. This information was obtained
from the study subjects in a 6-month post-treatment phone interview conducted
by one of the study coordinators. During all visits herpetic lesions were
morphologically staged (as papules, vesicles, crusts, or healed), lesion
counts and measurements taken, and patients were asked to rate their subjective
symptoms (which included pain, pruritus, burning, and stinging). Patient
serum was collected on day 3 to determine serum electrolytes, liver function
tests, hematologic profiles as well as DMSO levels. DMSO serum levels
were measured by gas chromatography at the National Medical Services Laboratory,
Willow Grove, Pa., USA. Specimens for virus isolation were taken on days
1, 3, and 5 by the methods used by Friedman-Klein et al.15Statistics
were prepared by ![]() 2
analysis and Student's t test where applicable.
2
analysis and Student's t test where applicable.
Of the 40 patients who initially enrolled in this patient-initiated clinical trial and received tubes of medication, 20 completed therapy of one HSV recurrence (12 medicated, 8 placebo). Of these 20, 19 individuals continued participation in the study through the 60- to 90-day posttreatment follow-up visit.The patients in the interferon-treated group demonstrated decreased viral shedding and more rapid healing than those in the placebo group. 66% of the interferon-treated patients were culture negative after 24 h of treatment (day 1), whereas only 25% of the patients in the placebo group were culture negative at that time (p<0.02). By day 5, 91% of the interferon group was culture negative compared to 85% of the placebo group (p > 0.66; fig. 1). On day 5, 25% of the patients in the placebo group had active lesions, while no patients in the interferon group had active lesions (p > 0.02).
Patients presenting with a negative viral isolation text.
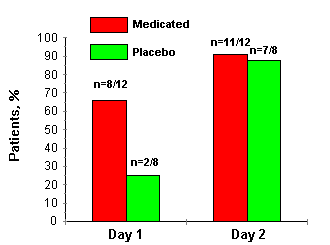
After completion of therapy, the patients in the placebo group had twice as many recurrences over the ensuing 6 months as did their counterparts who had received interferon ointment. Furthermore, the recurrences in the placebo-treated group took 2.3 days longer to heal than recurrences in the interferon group (p>0.10; table 1). At the end of the study, the patients were asked whether or not they believed that their treatment had been useful. Eleven of the 12 patients (92%) in the interferon group rated their treatment as 'useful.' Only 1 of 8 patients (12%) in the placebo group regarded treatment as 'useful' (p<0.003). The physician's global evaluation corresponded rather closely with that of the patients. Investigators rated therapy as worthwhile in 11/12 (92%) of individuals in the interferon group versus 2/8 (25%) in the placebo group (p<0.01).
Average number of recurrences (after treatment)
| Medicated | Placebo | |
|---|---|---|
| Mean | 1.18 | 2.25 |
| Sd | 0.83 | 1.39 |
| Number | 11 | 8 |
| Minimum | 0 | 0 |
| Maximum | 2 | 5 |
| t | -1.93 | |
| d.f. | 17 | |
| p | < 0.10 | |
| Median | 1 | 2 |
These results further support our belief that topical interferon preparations
mav have a role in the treatment of recurrent HSV infections.13-18
Topical interferon could be used in place of systemic acyclovir in patients
who dislike taking oral medications. Additionally, a topical agent, such
as the one evaluated in this study or our earlier clinical trial,12
could be used as an adjunct along with systemic acyclovir to minimize
the emergence of acyclovir-resistance strains of HSV.8,32-43One
area of concern is the high dropout rate in this clinical trial. Only
20/40 study subjects completed therapy of one recurrent episode. This
50% dropout rate is not unusually high for a patients-initiated study
for recurrent herpes. In 1983, Fiddian et al.19
reported that 62% of the 137 patients enrolled in their acyclovir cream
study for recurrent genital HSV completed that clinical trial. In a larger
multicenter study evaluating acyclovir ointment in recurrent genital herpes,
56% of the 547 entering patients completed therapy.23
In another multicenter clinical trial testing topical acyclovir in recurrent
oral HSV, Spruance et al.44 reported that only
20% of the 352 enrollees finished the study. Subsequently, our 50% dropout
rate does not seem alarmingly high for a patient-initiated study of this
type.Based upon published studies evaluating a variety of topical interferon
preparations in recurrent facial and Genital HSV infections, it is not
possible to select an optimal preparation. The topical ![]() -interferon
cream tested by Israeli investigators16-18 and the topical
-interferon
cream tested by Israeli investigators16-18 and the topical
![]() -interferon
preparations evaluated by us and Friedman-Kien et al.14.15
were all similarly impressive in reducing the duration of viral shedding
and of subjective symptoms. In the present clinical trial and in the study
by Glezerman et al.,6 there was an impressive
decrease in the recurrence rate and in the seventy of subsequent recurrences
after the study terminated and use of topical interferon was discontinued.
This can be just as easily attributed to the small study population in
both clinical trials (40 patients in the present study and 25 in the report
of Glezerman et al.) as to any long-term, positive residual pharmacologic
effect from interferon.As described under Materials
and Methods, the preparation tested contained DMSO. This was added
to enhance the penetration of interferon.19-21 Although
we cannot unequivocally exclude the possibility that DMSO enhances the
antiviral activity of interferon, we can confidently assert that DMSO
absorption was not significant and that there was no systemic toxicity
(as shown by DMSO drug levels, blood chemistries, hematologic profiles,
and physical examination).In summary, we feel that topical
-interferon
preparations evaluated by us and Friedman-Kien et al.14.15
were all similarly impressive in reducing the duration of viral shedding
and of subjective symptoms. In the present clinical trial and in the study
by Glezerman et al.,6 there was an impressive
decrease in the recurrence rate and in the seventy of subsequent recurrences
after the study terminated and use of topical interferon was discontinued.
This can be just as easily attributed to the small study population in
both clinical trials (40 patients in the present study and 25 in the report
of Glezerman et al.) as to any long-term, positive residual pharmacologic
effect from interferon.As described under Materials
and Methods, the preparation tested contained DMSO. This was added
to enhance the penetration of interferon.19-21 Although
we cannot unequivocally exclude the possibility that DMSO enhances the
antiviral activity of interferon, we can confidently assert that DMSO
absorption was not significant and that there was no systemic toxicity
(as shown by DMSO drug levels, blood chemistries, hematologic profiles,
and physical examination).In summary, we feel that topical ![]() -interferon
is a potentially safe and useful therapy for recurrent herpes genitalis.
It might be utilized adjunctively along with oral acyclovir or as monotherapy,
either during recurrences or by asymptomatic carriers between episodes
to diminish the emergence of resistant strains of HSV.
-interferon
is a potentially safe and useful therapy for recurrent herpes genitalis.
It might be utilized adjunctively along with oral acyclovir or as monotherapy,
either during recurrences or by asymptomatic carriers between episodes
to diminish the emergence of resistant strains of HSV.
- Gonzolez E.: Sexually transmitted diseases: in Fitzpatrick T.B., Eisen A.Z.. Wolff K., Freedberg I.M., Austen K.F. (eds): Dermatology in General Medicine. New York, McGraw-Hill, 1987, vol. 2, pp 2385-2467.
- Burnett J.W., Crutcher W.A.: Viral and rickettsial infections: in Moschella S.L. Hurley H.J. (eds): Dermatology. Philadelphia. Saunders. 1985, vol 1. pp 681-683.
- Mindel A. Faherty A. Hindley D. et al: Prophylactic oral acyclovir in recurrent genital herpers. Lancet 1984:ii:57-59.
- Fiddian A.P., Halsos A.M., Kinge B.R., et al: Oral acyclovir in the treatment of genital herpes. Proceedings of a symposium on acyclovir sponsored by Burroughs Welcome. Am J Med 1982;73;335-337.
- Straus S.E., Takiff H.E., Seidlin M.: Suppression of frequently recurring genital herpes: A placebo-controlled double-blind trial of oral acyclovir. N Engl J Med 1984:310:1545-1550.
- Bryson Y.J., Dillon M., Lovett M.: Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. N Eng J Med 1983:308:916-921.
- Douglas J.M., Critchlow M.S., Benedetti J.: A double-blind study of oral acyclovir for suppression of recurrences of genital herpes simplex virus infection. N Engl Med 1984:310:1551-1556.
- Straus S.E., Cruen K.D., Sawyer M.H., et al: Acyclovir suppression of frequently recurring genital herpes. Efficacy and diminishing need during successive years of treatment. JAMA 1988:260:2227-2230.
- Mertz G.J., Lawrence E., Kaufman R., et al: Prolonged continuous versus intermittent oral acyclovir treatment in normal adults with frequently recurring genital herpes simplex virus infection. Am J Med 1988:85:14-19.
- Kaplowitz L.G., Baker D., Gelb L., et al: Prolonged continuous acyclovir treatment of normal adults with frequently recurring genital herpes simplex vius infection. Am J Med 1988:85:14-19.
- Straus S.E., Mindell S., Takiff H.E., et al: Effect of oral acyclovir treatment on symptomatic and asymptomatic virus shedding in recurrent genital herpes. Sex Transm Dis 1989:16:107-112.
- Pazin G.J., Armstrong J.A., Lam M.T.: Prevention of reactivated herpes simplex infection by human leukocyte interferon after operation on the trigeminal rooot. N Engl J Med 1979:301:225-230.
- Asculai S.S., Weis M.T., Rancourt M.W., et al: Inactivation of heres simplex viruses by nonionic surfactants. Antimicrob Agents Chemother 1978:13-686-690.
- Shupack J., Stiller M., Knobler E., Ackerman C., Jondreau L., Kenny C.: Topical alpha-infection. A double-blind, placebo-controlled clinical trial. Dermatologica 1990:181:134-138.
- Friedman-Kein A.E., Klein R.J., Glaser R.D.: Treatment of recurrent genital herpes with topical alpha interferon get combined with non-oxynol-9. J Am Acad Dermatol 1986:15:989-994.
- Glezerman M., Cohen V., Movshovitz M., et al: Placebo controlled trial of topical interferon in labial and genital herpes. Lancet 1988:i:150-152.
- Movshovitz M., Schwach-Millet M., Kriss-Leventon S.,
et al: Topical treatment of recurrent facial and genital herpes
with human interferon
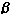 cream; in Kono R. (ed): Herpes Virus Chemotherapy. Amsterdam.
Elsevier Science Publishers. 1985, pp. 285-292.
cream; in Kono R. (ed): Herpes Virus Chemotherapy. Amsterdam.
Elsevier Science Publishers. 1985, pp. 285-292.
- Isacsohn M., Berson B., Sternberg I., Murag A.: Human fibroblast interferon treatment of viral diseases of the skin and mucous membranes. Isr J Med Sci 1983:19:959-962.
- Fiddian A.P., Kinghorn G.R., Goldmeier D., et al: Topical acyclovir in the treatment of genital herpes: A comparison with systemic therapy. J Antimicrob chemother 1983:3:291-301.
- Kinghorn G.R., Turner E.B., Barton I.G., et al: Efficacy of topical acyclovir cream in first and recurrent episodes of genital herpes. Antiviral Res 1983:3:291-301.
- Fiddian A.P., Brigden D., Yeo J.M., et al: Acyclovir: An update of the clinical applications of this antiherpes agent. Antiviral Res 1984:4:99-117.
- Kinghorn G.R.: Topical acyclovir in the treatment of recurrent herpes simplex virus infections. Scand J Infect Dis 1985:suppl 47:58-62.
- Luby J.P., Gnann J.W., Alexander J.W., et al: A collaborative study of patient-initiated treatment of recurrent genital herpes with topical acyclovir or placebo. J Infect Dis 1984:150:1-6.
- Reichman J., Badger G.J., Guinan M.E., et al: Topically administered acyclovir in the treatment of recurrent herpes simplex genitalis: A controlled trial. J Infect Dis 1983:147:336-340.
- Corey L., Nahmias A.J., Guinan M.E., et al: A trial of topical acyclovir in genital herpes simplex N Engl J Med 1982:306:1313-1319.
- Freeman D.J., Sheth N.V., Spruance S.L.: Failure of topical acyclovir in ointment to penetrate human skin. Antimicrob Agents Chemother 1986:29:730-732.
- Barton I.G., Kinghorn G.R., Rowland M., et al: Recurrences after first episodes of genital herpes in patients treated with topical acyclovir cream. Antiviral Res 1984:4:293-300.
- Fawcett H.A., Wansbrough-Jones M.H., Clark A.E., et al: Prophylactic topical acyclovir for frequent recurrent herpes simplex infection with and without erythema multiforme. Br Med J 1983:287:798-799.
- Morrison R.T., Boyd R.H.: Organic Chemistry. Newton, Allyn, and Bacon. 1983. pp. 33-34.
- Fisher A.A.: Dimethyl sulfoxide as a vehicle for food allergy patch tests. Cutis 1986:42:109-110.
- Goldman L., Igelman J.M., Kitzmiller K.: Investigative studies with DMSO in dermatology. Ann NY Acad Sci 1967:141:429-436.
- Erlich S.K., Mills J., Chatis P., et al: Acyclovir resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med 1988:320:293-296.
- Chatis P.A., Miller C.H. Schrager L.E. Crumpacker C.S.: Successful treatment with foscarnet of an acyclovir resistant mucocutaneous infection with herpes simplex virus in a patient with acquired immunodeficiency syndrome. N Engl J Med 1989.320:297-300.
- Burns W.H., Santos G.W., Soral R., et al: Isolation and characterization of resistant herpes simplex virus after acyclovir therapy. Lancet 1982:i:421-423.
- Wade J.C., McLaren C., Myers J.D.: Frequency and significance of acyclovir-resistant herpes simpiex virus isolated from marrow transplant patients receiving multiple courses of treatment with acyclovir. J Infect Dis 1983:148:1077-1082.
- Schinazi R.F., del Bene V., Scott R.T., Dudley-Thorpe J.B.: Characterization of acyclovir-resistant and sensitive herpes simplex viruses from a patient with an acquired immune deficiency. J Antimicrob Chemother 1986,18(suppl):127-134.
- Swennerholm B., Vohline A., Lowhogen G.B., et al: Sensitivity of HSV strains isolated before and after treatment with acyclovir. Scand J Infect Dis 985:47:149-154.
- Crumpacker C.S., Schnipper L.E., Marlowe S.I., Kowalsky P.N., Hershey B.J., Levin M.J.: Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med 1982:306:343-346.
- Crumpacker C: Significance of resistance of herpes simplex virus to acyclovir. J Am Acad Dermatol 1988;18:190-195.
- Ellis M.N., Keller P.M., Fyfe J.A., et al: Clinical isolate of herpes simplex type 2 that induces a thymidine kinase with altered substrate specificity. Antimicrob Agents Chemother 1987;31:1117-1125.
- Dekker C., Ellis M.N., McLaren C., et al: Virus resistance in clinical practice. J Antimicrob Chemother 1983;12(suppl):137-152.
- Burns W.E., Santos G.W., Soral R., et al: Isolation and characterization of resistant herpes simplex virus after acyclovir therapy. Lancet 1982:i:421-423.
- Wade J.C., McLaren C., Myers J.D.: Frequency and significance of acyclovir-resistant herpes simplex virus isolated from marrow transplant patients receiving multiple courses of treatment with acyclovir. J Infect Dis 1983.148:1077-1082.
- Spruance S.L., Crumpacker C.S., Schnipper L.E., et al: Early, patient-initiated treatment of herpes labialis with topical 10% acyclovir. Antimicrob Agents Chemother 1984.23:553-555.
Pharmacology and Treatment, Dermatology 1992;184:40-44
This clinical trial was supported by a grant from Virapen Corporation.
Jerome Shupack, MD
Department of Dermatology
New York University Medical Center
562 First Avenue, New York, NY 10016 (USA)© 1992 Karger AG. Base
1018-8665/92/1841-0040
$ 2.75/0
DMSO Organization thanks the Dermotology publishers for allowing this article to be placed on our World Wide Web Site. The publisher retains all copyright. Please contact the publisher for permission to copy any portion of this article.
© 2001-2022 All rights reserved